U.K. approves CRISPR gene editing to create GMO humans
11/30/2023 / By Ethan Huff
Regulators in the United Kingdom have approved the world’s first CRISPR therapy to treat humans – and United States regulators are expected to soon follow.
The gene-editing therapy, which was reportedly designed to treat blood disorders, could appear on the market by as soon as December.
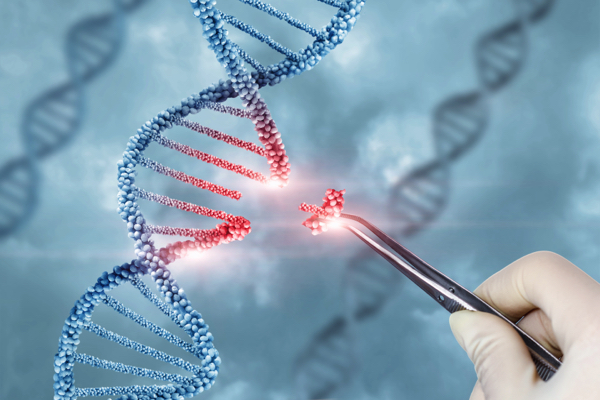
The way CRISPR works is that it functions like a pair of “genetic scissors,” chopping up sections of DNA so scientists can edit them by “snipping” specific portions and replacing those portions with new, artificial segments.
According to a 2012 paper that first announced the technology, CRISPR is basically a cheap and easy way to edit genes and create new genetically modified organisms (GMOs) – in this case, out of human beings.
Known as exa-cel therapy, the new CRISPR about to be released, known formally as Casgevy, is the world’s first CRISPR therapy for humans to receive approval on the market.
CRISPR’s inventers won a Nobel Prize in chemistry in 2020 for the discovery, which in recent years has been used to manipulate plants as a possible solution to “climate change.” Only now is CRISPR about to be used on humans, despite the fact that it is highly risky.
(Related: Gene-edited salad greens are also coming soon to a grocery store near you – and no, they will not be labeled.)
Is genetically modifying your body really worth a “cure?”
Casgevy was designed to treat two specific blood conditions: sickle cell disease and beta thalassemia. Also known as sickle cell anemia, the former most commonly occurs in people of African or Caribbean descent and is known to cause debilitating pain.
Beta thalassemia, which can cause mild or severe anemia, is a similar condition that typically requires regular blood transfusions to treat.
Both of these genetic conditions are said to be caused by “errors” in the genes that regulate hemoglobin, a protein that lets red blood cells transport oxygen around the body. Both conditions can also be fatal if left untreated.
Developed by Vertex Pharmaceuticals and CRISPR Therapeutics, the technology was approved after just one study that followed only 29 out of 45 total participants for 16 months. Twenty-eight out of these 29 supposedly had no pain after one year, Nature reported.
In a clinical trial for beta thalassemia, 39 of the 42 trial participants tracked did not end up needing a routine red blood cell transfusion for a full 12 months at least – without Casgevy, these same beta thalassemia patients require a blood transfusion every three to five weeks.
While the media and the companies they back that produce this kind of stuff are celebrating these amazing recoveries, what they are not telling people is that CRISPR comes with serious side effects, not the least of which include unexpectedly large deletions that result in even more serious genetic mutations.
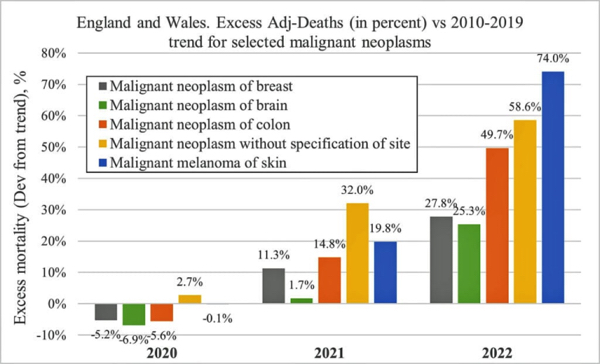
“It is well established that CRISPR / Cas9 gene editing is not only prone to off-target genetic damage but also a wide range of unintended mutations even at the intended edit site,” said Michael Antoniou, PhD., head of the Gene Expression & Therapy Group at King’s College London. “This can negatively impact the function of multiple genes, which can lead to cancer.”
“It is thus vital that those responsible for administering the gene editing therapy conduct unbiased genome-wide analysis of treated patients for potentially life-damaging DNA mutations. This is essential as it only takes one cell within the large pool of edited cells to go wrong and cause cancer.”
In the meantime, a U.S. company is pushing a different but similar CRISPR technology called “base editing” that was reportedly designed to artificially reduce people’s cholesterol levels.
More related news coverage about the genetic modification of humans can be found at GeneticLunacy.com.
Sources for this article include:
Submit a correction >>
Tagged Under:
Big Pharma, biotechnology, cancer criminals, Casgevy, CRISPR, dangerous, exa-cel therapy, genetic engineering, genetic lunacy, Genetic Mutations, GMO, Great Britain, health science, United Kingdom
This article may contain statements that reflect the opinion of the author
RECENT NEWS & ARTICLES
COPYRIGHT © 2017 RESEARCH NEWS